Introduction
In my 20 years working in beverage machinery and water treatment, I’ve noticed one recurring issue: customers often get confused when deciding between reverse osmosis (RO) and nanofiltration (NF) systems. On paper, both look like “membrane filtration,” but in reality, the choice can make or break your beverage product’s taste, stability, and even production cost.
Just a few weeks ago, an Argentine client came to us with an unusual request. He wanted to use natural spring water as the source for carbonated soft drinks. Normally, when a client tells me they have access to spring water, my first thought is bottled mineral water—because many brands use the natural mineral profile of spring water as a selling point. But when it comes to carbonated beverages, the rules are different. This situation is a perfect example of why understanding the differences between RO and NF matters.
What is Reverse Osmosis (RO)?
Think of RO as a reset button for water quality. By applying high pressure, RO forces water through a semi-permeable membrane with extremely small pores (around 0.0001 microns). The result is almost pure water, with 95–99% of dissolved salts, organics, and microorganisms removed.
Based on my past experience, I’ve seen RO systems deliver water so consistent that even if the raw water fluctuates in quality, the product line remains stable. That’s why major carbonated drink brands rely on RO—they need the water to be a blank canvas so their syrup and CO₂ behave predictably.
Key advantages of RO in beverage production:
- Produces nearly mineral-free water, eliminating the risk of unwanted chemical reactions.
- Ensures carbonation efficiency—no surprise foaming or sedimentation.
- Provides microbiological safety without relying only on chemical disinfectants.
If your final product is cola, soda, or flavored sparkling water, RO is usually the safest bet.
What is Nanofiltration (NF)?
NF, on the other hand, is like fine-tuning the water instead of resetting it. With slightly larger pores (~0.001 microns), NF removes hardness ions like calcium and magnesium but allows some monovalent ions (sodium, potassium) to remain.
This means the water is softer and safer, but still retains a natural mineral taste. That’s exactly why NF is popular with bottled water producers. A customer once told me:
“If we strip everything out, then my mineral water just becomes purified water. Why would consumers pay a premium?”
Key advantages of NF in beverage production:
- Preserves part of the mineral profile, giving water a more natural taste.
- Reduces hardness, protecting pipes and machines from scaling.
- Operates at lower pressure than RO, saving energy in long-term operations.
If your product is bottled mineral water where the label proudly says “from natural spring sources,” NF is often the right choice.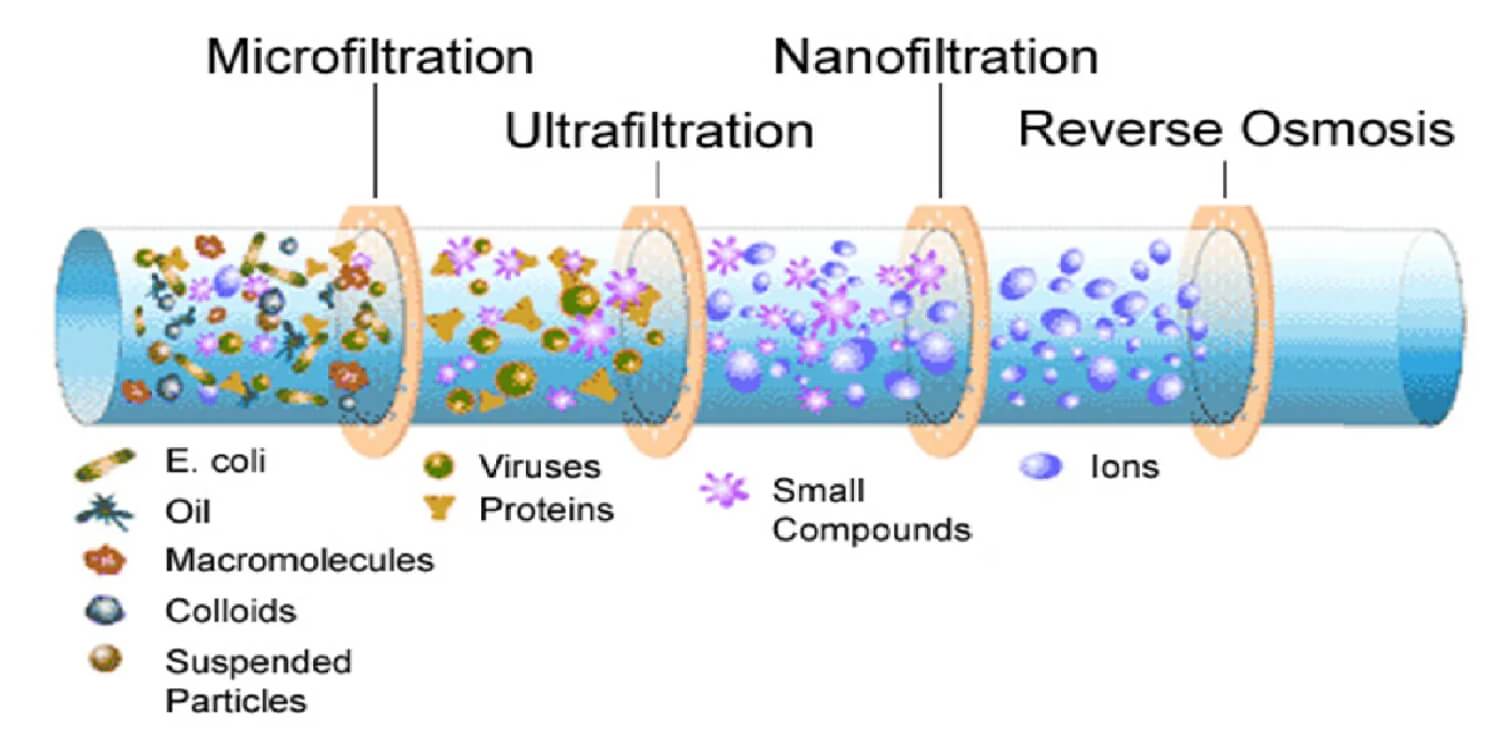
RO vs NF: Practical Differences
| Feature | Reverse Osmosis (RO) | Nanofiltration (NF) |
| Salt rejection | 95–99% | 50–80% |
| Mineral retention | Almost none | Partial (taste preserved) |
| Taste profile | Neutral, blank canvas | Natural, mineral-rich |
| Operating pressure | Higher | Lower |
| Energy consumption | Higher | Lower |
| Best for | Carbonated & flavored drinks | Bottled mineral water |
Lessons from the Argentine Client
Let’s go back to my Argentine client. He wanted to bottle carbonated soft drinks using spring water. His first thought was: “Spring water is already clean, why not just filter and use it?”
Here’s the challenge: spring water might be clean microbiologically, but its mineral composition varies. High calcium or magnesium levels can react with carbonation, leading to cloudy drinks, scaling inside machines, or inconsistent taste across batches. That’s unacceptable for a carbonated product.
After running water analysis and discussing his product goals, my recommendation was clear: go with RO. It would give him the stable, mineral-free base he needed for carbonation. Yes, it meant removing the natural spring water “character,” but for his product category, consistency was more valuable than branding the mineral content.
This case illustrates a point I always emphasize to customers: the right technology isn’t about what sounds better on paper, but what works best for your product line.
How Should You Decide?
When advising engineers and procurement managers, I suggest asking four simple questions:
- What is the raw water quality?
– If TDS and hardness are high, RO might be necessary. If they’re moderate, NF could work. - What is the final product?
– Carbonated or flavored drinks → RO. Bottled spring water → NF. - What do consumers expect?
– If they want natural minerals, NF is the way to go. If they expect a neutral base, RO wins. - What’s the long-term cost tolerance?
– RO consumes more energy but can prevent downstream problems. NF saves energy but may not meet all product requirements.
Conclusion
In water treatment, there’s no one-size-fits-all solution.
- RO is ideal for carbonated and flavored beverages, where water must be stable, neutral, and predictable.
- NF is ideal for bottled mineral water, where preserving part of the natural character is a selling point.
My advice to beverage producers is this: don’t choose based only on what your competitor uses—choose based on your product’s chemistry and your customer’s expectations.
If you’re not sure which direction to take, start with a water analysis and a clear definition of your end product. From there, we can design a system—RO, NF, or even a hybrid—that ensures both technical reliability and market appeal.
After two decades in this industry, I can tell you one thing with confidence: the cost of choosing the wrong technology is always higher than investing in the right one from the start.
